Spectrometer Franz Schmidt & Haensch in Berlin
Spectrometer made by Firm „Franz Schmidt & Haensch", Berlin
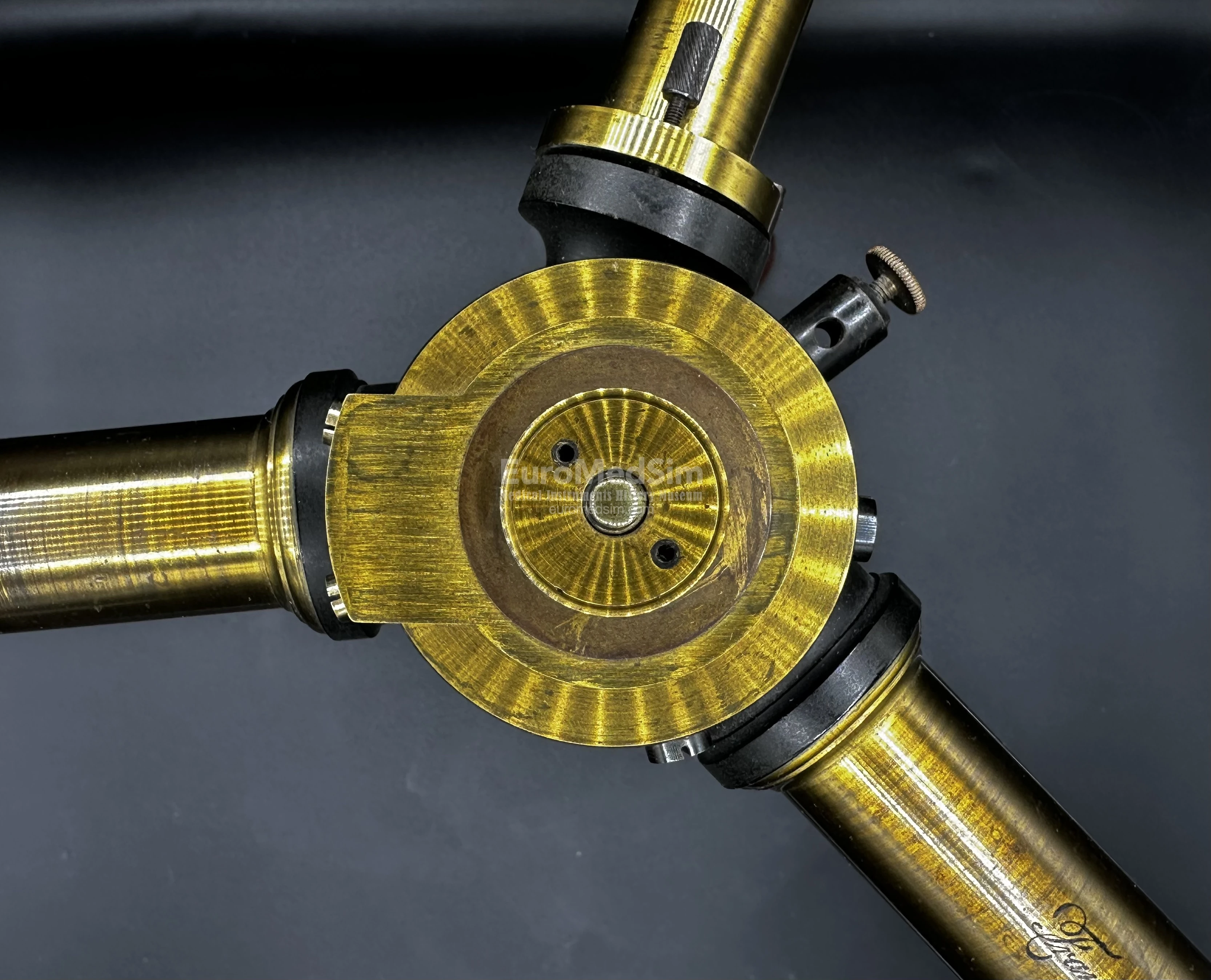
A large and impressive spectrometer (sometimes referred to as a spectroscope; German: Spektrometer) manufactured by the firm Franz Schmidt & Haensch, Berlin, in the late 19th century. Constructed of lacquered and ebonized brass, it features a brown enamelled tripod base made of cast iron. The main assembly consists of a central pillar mounted on the tripod base, supporting a drum that holds the prism and three tubes. These include:
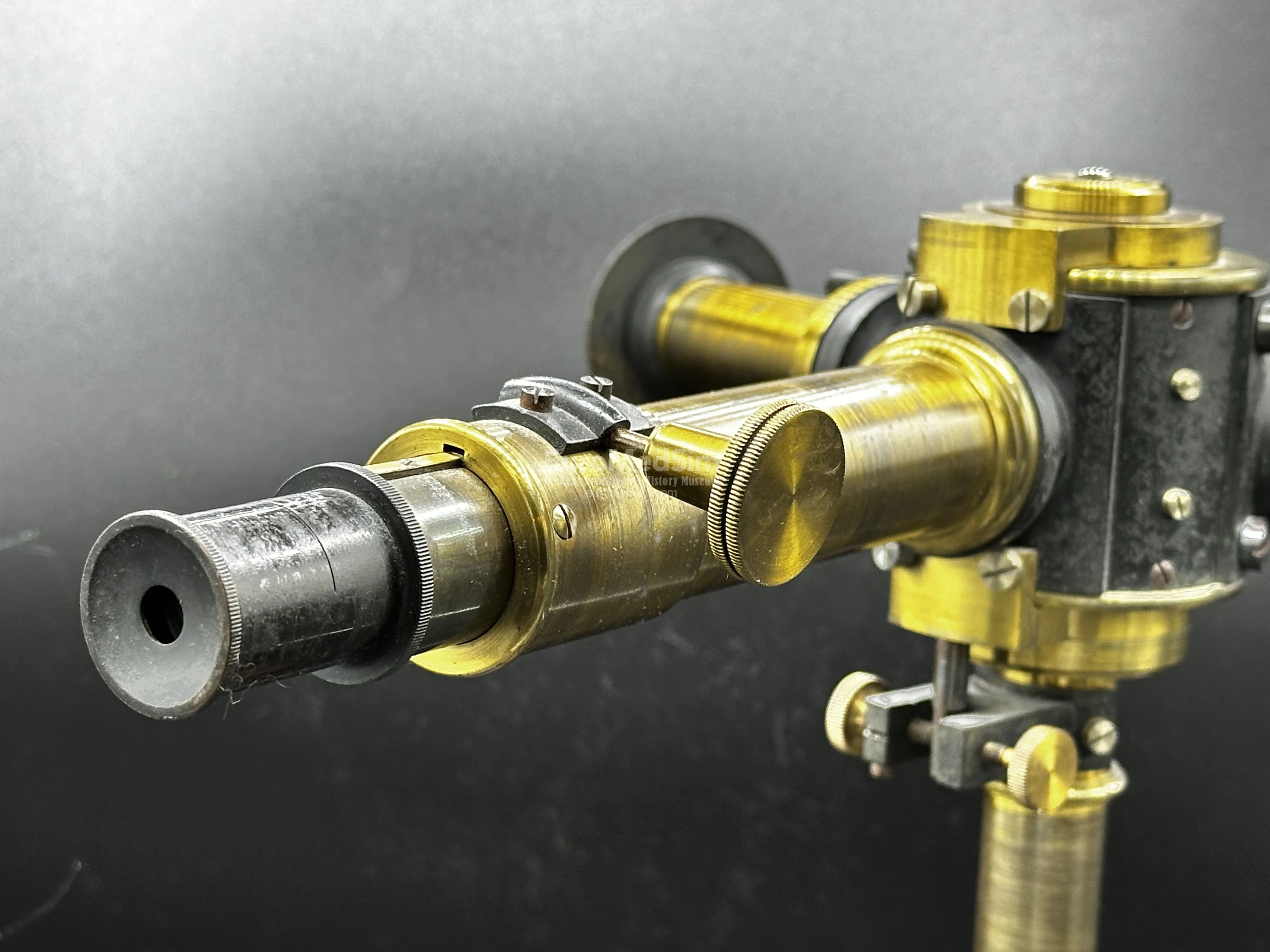
- An observation telescope, equipped with a screw adjustment for fine focusing. The rounded connection of the telescope to the drum allows you to change the angle at which the optical tube is directed to the prism. Smooth adjustment is accomplished by means of two oppositely directed small screws on the center pillar.
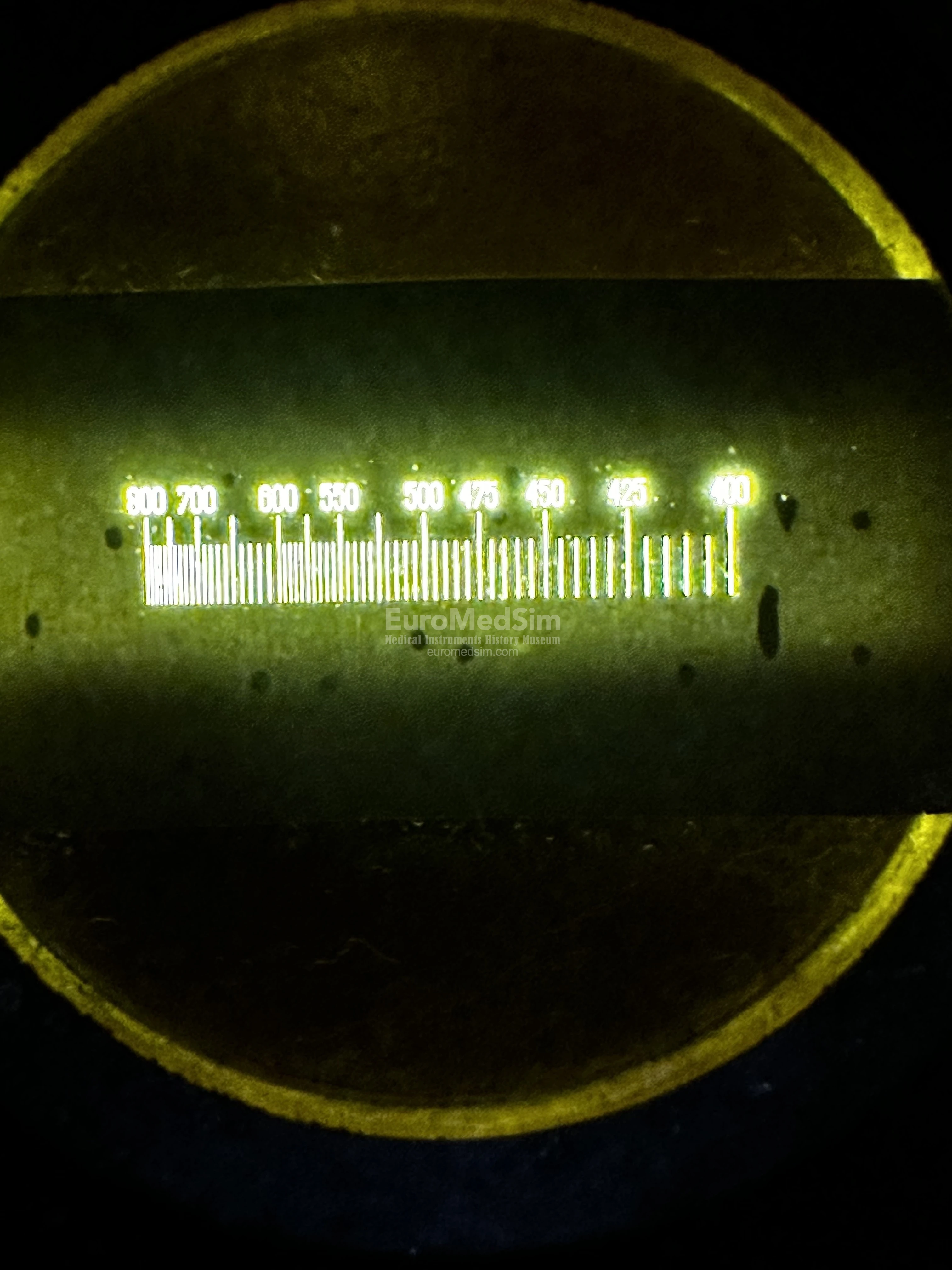
- An auxiliary tube fitted with a micrometer eyepiece (an ocular lens engraved with a scale ranging from 400 to 800), designed for the direct measurement of spectral line positions or wavelengths.
- A collimator tube with a narrow, adjustable slit, regulated by a precision brass screw. An elegant italic inscription reading "Franz Schmidt & Haensch, Berlin." is engraved on the upper side of the collimator tube.
Height 33 cm, length of collimator 15.2 cm, max. length of telescope — 18.7 cm. Weight 2688 g.
A Tool of Science and Medicine
By the end of the 19th century, the spectrometer had become an indispensable instrument in both physics and medicine. Emerging from earlier prism experiments by Newton and Fraunhofer, the device evolved into a precise tool for analyzing light, enabling breakthroughs in chemistry, astronomy, and medical diagnostics. The spectrometer in this museum collection represents a key technological advancement of its time, bridging laboratory science and practical medical applications.
The modern spectrometer traces its origins to numerous outstanding scientists — Isaac Newton, Joseph von Fraunhofer, William Hyde Wollaston. German physicists Gustav Kirchhoff and Robert Bunsen demonstrated in 1860s that each chemical element emits or absorbs light at specific wavelengths, creating unique spectral lines — that was the beginning of the chemical spectral analysis. By the late 1800s, spectrometers had become more sophisticated, featuring adjustable slits for precise light control, calibrated wavelength scales for accurate measurement, photographic plates to record spectra permanently.
These improvements allowed scientists to identify unknown substances, discover new elements (such as helium in the Sun’s spectrum), and study molecular structures.
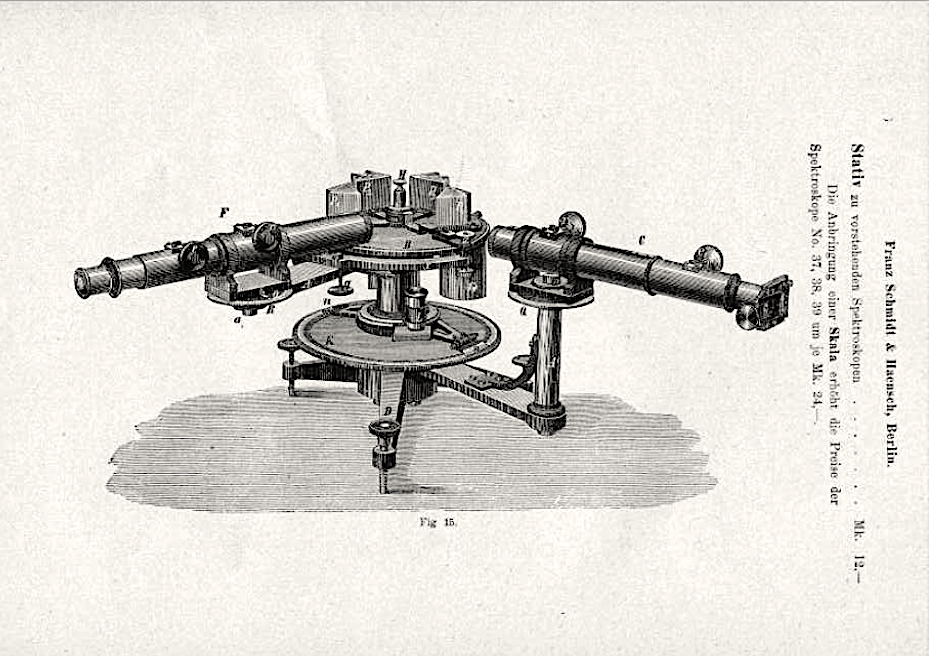
Page 16, Catalogue of the optical instruments and utensils exhibited by the company Franz Schmdit und Haensch Werkstäten für Präzisions-Mechanik und Optik (Berlin S. Stallschreiber-Str. 4) at the Berlin Trade Exhibition in 1896. Source: Full-text-link at Osservatorio Astronomico di Palermo
The principle of use
A late 19th-century spectrometer analyzed light by breaking it into its component wavelengths (colors) to identify chemical substances. Here are main components:
- Light Source – A flame (e.g., Bunsen burner) or electric arc heated a sample, causing it to emit light.
- Collimator – A narrow slit focused the light into a beam, which passed through a lens to make it parallel.
- Prism (or Diffraction Grating) – The light then hit a prism, splitting into a spectrum of distinct colored lines.
- Telescope & Scale – An adjustable telescope magnified the spectrum, while a calibrated scale measured the angles of spectral lines.
- Detection – The observer recorded the pattern (either by eye or on photographic plates) and matched it to known elemental spectra (e.g., sodium’s yellow line).
Mostly used in Chemistry, spectrometry has found its place in medicine as well. Doctors and chemists used this to detect toxic metals (like arsenic in poison cases) or analyze blood/urine for disease markers by their unique spectral "fingerprints." This simple but revolutionary process laid the foundation for modern chemical and medical analysis.
Medical Applications of Spectroscopy
The late 19th-century spectrometer laid the groundwork for today’s spectrophotometers and mass spectrometers, which are essential in labs worldwide. While this antique model seems rudimentary now, it embodies a pivotal moment when medicine began embracing biochemical analysis, forever changing diagnostics and research. In medicine, spectrometers were used for:
- Toxicology & Forensic Analysis – Detecting poisons (e.g., arsenic) by their spectral signatures.
- Blood and Urine Analysis – Identifying abnormal compounds linked to diseases.
- Bacteriology – Studying microbial metabolism by analyzing chemical byproducts.
Though less common than microscopes in clinics, spectrometers provided objective, chemical evidence that complemented traditional diagnostic methods.
See article on the company-manufacturer: Schmidt + Haensch, Berlin
Provenance
Acquired from the Bavarian Scientific collection through the Auctionshaus LANKES in 2021
References
-
Kirchhoff Bunsen-type spectroscope, ca. 1900 made by A. Krüss Hamburg
- Hilger wavelength spectroscope, with camera, London, England, 1919. Science Museum, London, UK
- Franz Schmidt & Haensch Spectroscope (Germany, 1903) at antiguedades.es
- Catalogue of the optical instruments and utensils exhibited by the company Franz Schmdit und Haensch Werkstäten für Präzisions-Mechanik und Optik (Berlin S. Stallschreiber-Str. 4) at the Berlin Trade Exhibition in 1896. Source: Osservatorio Astronomico di Palermo